SECTION 10-C 1954 BUICK BATTERY AND CABLES
10-14 GENERAL 1954 BUICK BATTERY INFORMATION
Delco-Remy 12-volt storage battery number 3 EM 60-W is used in all models. This battery has 6 cells with 9 plates per cell, a capacity of 60 ampere-hours at a 20 hour rate, and a rating of 720 watt-hours.
The 1954 Buick battery is mounted on the left front fender skirt under the hood. The 1954 Buick battery negative (-) post is grounded to the engine mounting bracket by a flexible copper cable. The positive (+) post is connected by an insulated copper cable to a junction block on the fender skirt. The cable terminal at the battery and junction block are protected by rubber boots.
Registration of 1954 Buick Battery
Delco-Remy Battery dealers and distributors are prepared to carry out terms of the manufacturer’s warranty on Delco-Remy batteries. In order that Buick owners shall have the protection and benefit of this warranty, it is necessary for the dealer or car owner to register his battery with the local Delco-Remy Battery dealer or distributor on all new car deliveries, and on all deliveries of new replacement Delco-Remy batteries.
Care of New Batteries in Storage
Batteries in stored new cars, as well as batteries in stock, must be given regular attention to prevent sulphation of plates that may result from inactivity and self-discharge. All automotive wet batteries will slowly discharge on standing idle, whether in stored vehicles or in stock, and will self-discharge much faster when warm than when cold. Batteries in stock should be rotated and the older ones used first.
To minimize the extent of self-discharge always store batteries fully charged and in the coolest possible place. At frequent intervals check the level of electrolyte and add water as required; also check the specific gravity with a hydrometer (par. 10-16). A boosting charge at a moderate rate (par. 10-19) without excessive overcharge, must be given batteries in storage whenever the specific gravity falls to 1.220, corrected for temperature. Batteries used for display purposes or standing in cars in storage must be treated in the same manner as batteries in stock.
When a new car, or a new replacement battery is delivered, make certain that it is fully charged and the electrolyte is at proper level. This is extremely important because the delivery of a partially discharged battery may not only lead to its return for charging but may also result in shortened life of 1954 Buick battery.
Importance of Maintaining Electrolyte at Proper Level
Water is the only component of the 1954 Buick battery which is lost as the result of charging and discharging, and it must be replaced before the electrolyte level falls to the tops of the separators. If the water is not replaced and the plates and separators become exposed, the acid will reach a dangerously high concentration that will char and disintegrate the wood separators and may permanently sulphate and impair the performance of the plates. Plates cannot take full part in the battery action unless they are completely covered by the electrolyte. Separators are no longer porous in the area that has once dried out as a result of exposure, therefore the corresponding area of adjoining plates is rendered inactive and subject to continuous sulphation.
Importance of Keeping 1954 Buick Battery Properly Charged
The 1954 Buick battery has three major functions: (1) It provides a source of energy for cranking the engine. (2) It acts as a stabilizer to the voltage in the electrical system. (3) It can for a limited time furnish energy when the demands of the electrical units in operation exceed the output of the generator.
In order for the 1954 Buick battery to continue to function, it is necessary that current withdrawal from the battery be balanced by current input from the generator so that the battery is maintained in a properly charged condition. If the outgo exceeds the input the battery will become discharged so that it cannot supply sufficient energy.
The state of charge of the 1954 Buick battery as well as the temperature of the electrolyte has an important bearing on its capacity for supplying energy. Battery efficiency is greatly reduced by decreased electrolyte temperature as it has a decided numbing effect on its electrochemical action. Under high discharge such as cranking, battery voltage drops to lower values in cold temperatures than in warm temperatures.
The following comparison shows how states of charge at temperatures of 80°, 32°, and 0° F. affect efficiency and cranking power, with efficiency in the fully charged state at 80° F. equal to 100%.
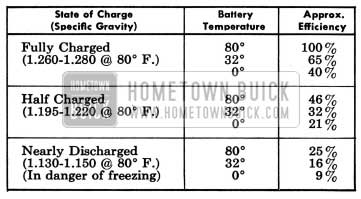
1954 Buick Battery Efficiency
In extremely cold climates it is important to keep batteries in a nearly full charged condition to avoid the possibility of freezing, which will damage any 1954 Buick battery. The following table shows the temperatures at which freezing will occur in electrolytes of different densities, with specific gravity corrected to 80° F.

1954 Buick Battery Freezing Point
10-15 PERIODIC 1954 BUICK BATTERY INSPECTION AND SERVICE
The 1954 Buick battery requires very little attention, but periodic inspection is essential to secure the maximum efficiency and life. The following services are essential to maintain the 1954 Buick battery at maximum efficiency:
CAUTION: The gas which is produced in the 1954 Buick battery cells during charging is dangerously explosive. Extreme care must be taken to avoid bringing open flames, lighted matches, etc., near a battery which is or has been recently on charge, and which is or has been gassing. Likewise care must be taken to avoid causing any sparks near a battery, since this can also set off an explosion of the gases. Before making contact with battery it is advisable to establish metallic contact between car bumper and ground to discharge any static charge in the car.
Maintain Electrolyte Level
Add distilled water as required to maintain the electrolyte level at the split ring at bottom of filler well. See figure 10-1.
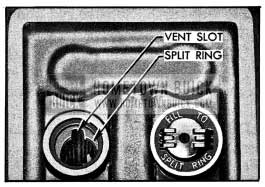
1954 Buick Battery Filler Well Lubricare
After filling, see that gaskets are in place when filler plugs are installed and tightened. CAUTION: Do not overfill, as electrolyte may be sprayed out by gassing or may overflow due to heat expansion during charging.
In freezing weather the water should be added just before using the car or otherwise charging the 1954 Buick battery so that the water will be mixed with the acid before it is allowed to stand in freezing temperatures.
If it is found necessary to add water to the battery more frequently than about every 1,000 miles and the quantity of .water added per cell is great, check setting of voltage regulator and adjust, if necessary (par. 10-26). Abnormal water loss is an indication that the 1954 Buick battery is being overcharged.
Inspect and Clean 1954 Buick Battery Mounting and Cables
Check the 1954 Buick battery hold down bolts to make certain that battery is securely held in place. The wing nuts should be drawn up finger tight; excessive tightening may distort or crack the 1954 Buick battery case.
If the top of 1954 Buick battery is dirty or the hold down strap is corroded, clean thoroughly with a brush dipped in ammonia or soda solution. Tighten vent plugs to prevent any solution from getting into battery cells. After the foaming of solution stops, flush off with clean water and dry thoroughly. If hold down strap is corroded it should be painted with acid-resisting paint after cleaning.
Check battery cables to make certain they are tight at 1954 Buick battery posts, engine mounting bracket and at Junction block. If a connection is found loose it should be cleaned before being tightened as arcing and corrosion may have taken place in the loose connection. Check condition of cables and replace if badly corroded or frayed. See paragraph 10-18 for instructions on cleaning and tightening cable terminals and replacement of cables.
Test 1954 Buick Battery for Internal Condition
- Hydrometer Test. It is advisable to occasionally check the condition of battery electrolyte with a hydrometer (par. 10-16) in order to determine whether the generating system is maintaining the battery in a proper state of charge. This is particularly important during cold weather.
- High Discharge Tests. It is advisable to occasionally check the internal condition of battery and the battery cables by means of high discharge test described in paragraph 10-17. This test will indicate whether the battery requires additional servicing, and whether the cables are setting up resistance to the free flow of current.
10-16 HYDROMETER TEST OF 1954 BUICK BATTERY
Specific Gravity and Effects of Temperature
The hydrometer measures the percentage of sulphuric acid in the battery electrolyte in terms of specific gravity. As a battery drops from a charged to a discharged condition, the acid leaves the solution and enters the plates, causing a decrease in specific gravity of electrolyte. By measuring specific gravity of the electrolyte with a hydrometer, an indication of the approximate state of charge of the 1954 Buick battery is obtained.
The specific gravity of the electrolyte varies not only with the percentage of acid in the liquid, it also varies with temperature. As temperature increases, the electrolyte expands so that the specific gravity is reduced. As temperature drops, the electrolyte contracts so that the specific gravity increases. Unless these variations in specific gravity are taken into account, the specific gravity obtained by the hydrometer may not give a true indication of the state of charge of 1954 Buick battery.
Correction for Temperature
Correction can be made for temperature by adding .004, usually referred to as 4 “points of gravity,” to the hydrometer reading for every 10° F. that the electrolyte is above 80° F. or subtracting .004 for every 10° F. that electrolyte is below 80° F. Figure 10-2 shows the exact correction figure to use for any temperature above or below 80° F., the three steps used in obtaining the corrected or true specific gravity, and two examples showing how it is figured.
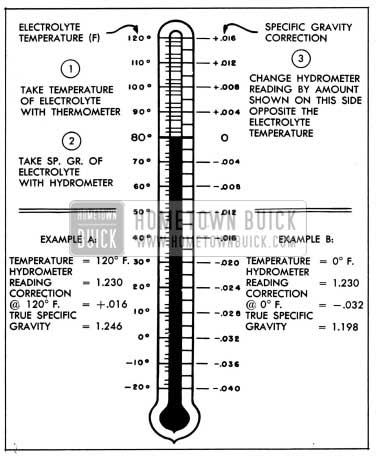
1954 Buick Specific Gravity Temperature Correction Scale
If the electrolyte temperature is not too far from the 80° F. standard, or if only an approximate idea of specific gravity reading is required, it will not be necessary to make the temperature correction. Hydrometers are available which have a built-in thermometer and temperature correction scale similar to figure 10-2. This type of hydrometer simplifies the operation of obtaining a true specific gravity reading.
Using Hydrometer
When using a hydrometer, observe the following points:
- Hydrometer must be clean, inside and out, to insure an accurate reading.
- Hydrometer readings must never be taken immediately after water has been added. The water must be thoroughly mixed with the electrolyte by charging for at least 30 minutes before hydrometer values are reliable.
- If hydrometer has built-in thermometer, draw liquid into it several times to insure correct temperature before taking a reading.
- Hold hydrometer vertically and draw in just enough liquid from battery cell so that float is free floating, and with bulb fully released. Hold hydrometer at eye level so that float is vertical and free of outer tube, then take reading at surface of liquid. Disregard the curvature where the liquid rises against float stem due to surface tension.
- Avoid dropping liquid on car or clothing as it is extremely corrosive. Any liquid that drops should be washed off immediately with soda solution.
Meaning of Specific Gravity Reading
The approximate state of charge of a battery as indicated by specific gravity corrected to 80° F. is as follows:
1.260-1.280 – Fully charged
1.225-1.250 – Three-fourths charged
1.195-1.220 – One-half charged
1.160-1.180 – One-fourth charged
1.130-1.155 – Barely operative
1.100-1.125 – Completely discharged
The specific gravity of a charged 1954 Buick battery should not vary more than 25 points (.025) between cells. A greater variation could be due to the low cell being shorted, to loss of electrolyte, or to the fact that the battery is old. Such battery should be removed for a slow charge and further tests.
10-17 HIGH DISCHARGE TEST OF 1954 BUICK BATTERY
The hydrometer test (par. 10-16) reports only the chemical condition of the 1954 Buick battery. A high discharge test with a voltmeter gives additional information on the condition of battery and its ability to deliver current under load. If the battery specific gravity is above 1.250 the high discharge test may be made.
A high discharge test of battery may be made at a battery temperature of 70-90°F. with equipment comprising a voltmeter (10 volts minimum), ammeter of 200 or more amperes capacity, and a carbon-pile rheostat having a minimum capacity of 200 amperes connected in series with the ammeter. This test may be made with battery either installed or removed, and without cranking the engine.
CAUTION: Gas formed in battery during charge is dangerously explosive. Sparks produced by test equipment may set off an explosion if gas remains in top of battery cells. If battery has been gassing recently, remove filler plugs, blow the gas out of cells and away from area, and install filler plugs before performing high discharge tests.
- Adjust rheostat to provide maximum resistance (OFF position).
- Connect ammeter positive (+) lead to 1954 Buick battery positive (+) post, and negative (-) lead to one side of rheostat. Connect other side of rheostat to 1954 Buick battery negative (-) post. In the instrument shown in figure 10-3 the ammeter and rheostat are connected in series inside the case. If 1954 Buick battery is installed, connect ammeter and rheostat leads to battery cable terminals so that battery posts are not covered.
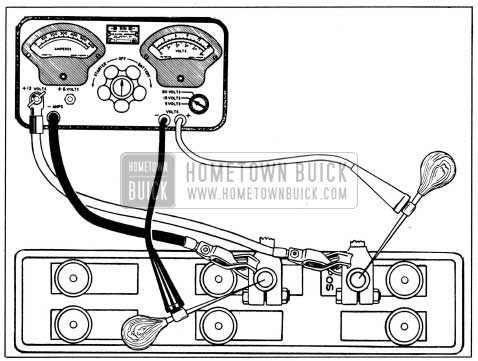
1954 Buick High Discharge Test Connections
10-18 TESTING AND CLEANING 1954 BUICK BATTERY AND CRANKING MOTOR CABLES
Whenever the 1954 Buick battery is tested (par. 10-17) the battery and cranking motor cables should also be inspected for condition and tested for resistance. Resistance in the cables and connections causes voltage drop, and excessive voltage drop is liable to cause starting difficulties.
Carefully inspect the 1954 Buick battery to junction block, battery to engine (ground) and cranking motor to junction block cables. If cable strands are broken, corroded, or loose in terminals the cable should be replaced with a genuine Buick cable to insure ample capacity.
Check terminals at both ends of each cable for tight connections. Since loose connections are usually dirty or corroded, any loose connections should be thoroughly cleaned before being tightened. If terminals are tight and cables are apparently in good condition, it is advisable to test them with a low-reading voltmeter to detect any abnormal internal resistance.
Testing Resistance of Cables and Terminal Connections
1954 Buick battery cables and terminal connections may be tested with equipment comprising a voltmeter (5 volts maximum), ammeter of 300 or more amperes capacity, and carbonpile rheostat having a minimum capacity of 300 amperes connected in series with the ammeter.
- Adjust rheostat to provide maximum resistance (“OFF” position).
- Connect ammeter positive (+) lead to battery terminal stud on junction block. Connect ammeter negative (-) lead to one side of rheostat and connect other side of rheostat to ground on engine, preferably at point where 1954 Buick battery ground strap is attached. In the instrument shown in figure 10-4 the ammeter and rheostat are connected in series inside the case.
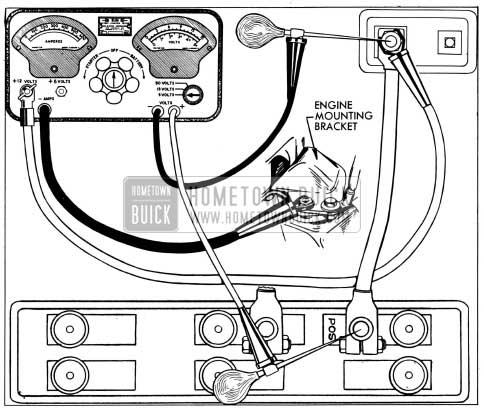
1954 Buick Battery Cable Test Connections
Cleaning Cable Terminals
If loose connections are found by inspection, or high resistance is found by voltage test, disconnect the cable for thorough cleaning of terminals. When removing a corroded cable terminal from battery post do not pry against 1954 Buick battery case or hammer on terminal to break it loose, since either practice will result in broken cell covers. Use a screw type terminal puller if terminal cannot be loosened by hand after clamp bolt is fully loosened.
Thoroughly clean all corrosion from disconnected battery cable terminals and terminal posts, using suitable wire brushes (fig. 10-5). If wire brushes are not available, corroded terminals may be cleaned by brushing with a strong soda solution, using care not to get solution into 1954 Buick battery cells.
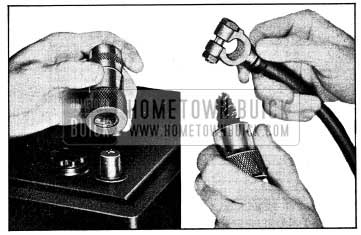
1954 Buick Wire Brushes for Cleaning Battery Posts and Terminals
To prevent corrosion of battery terminals and connections, apply a coating of petroleum jelly over the battery post and cable terminals.
CAUTION: Animal or vegetable greases will cause rapid corrosion of terminals. Make sure that rubber boots are in place over both terminals of battery to junction block cable.
Connecting 1954 Buick Battery Cables and Winding Clock
The electric clock requires special attention when reconnecting a 1954 Buick battery that has been disconnected for any reason. IT IS VERY IMPORTANT THAT THE INITIAL WIND BE FULLY MADE. See paragraph 10-56 for procedure.
10-19 1954 BUICK BATTERY RECHARGING
CAUTION: Occasionally, certain chemical “dopes” are offered which their sellers claim will “recharge” the 1954 Buick battery when put into the battery cells. Such chemical substances are harmful to the battery and must never be used. Use of such material voids all battery warranty. The only way to charge a battery is to connect it to a charging circuit.
There are two separate methods of recharging batteries which differ basically in the rate of charge. In the “slow-charge” method, the battery is supplied a relatively small amount of current for a relatively long period of time. In the “quick-charge” method, the 1954 Buick battery is supplied with a high current for a short period of time.
The “slow-charge” method, properly applied, may be safely used under all possible conditions of the battery, provided electrolyte is at proper level in all cells. The battery may be fully charged by this method, unless the battery is not capable of taking a full charge.
The normal charging rate for the 3 EM 60-W 12-volt battery is 4 amperes. If the battery electrolyte temperature is 60° F. or higher and the specified gravity is below 1.215, a charging rate of up to 40 amperes may be used until the specific gravity reaches 1.215, after which the rate must be tapered to 4 amperes. In no case should the electrolyte temperature be allowed to exceed 120° F. If the temperature reaches 120° F. charging must be suspended until the electrolyte cools, or serious damage to the battery plates will result.
A 1954 Buick battery cannot be brought up to a fully charged condition by the “quick-charge” method. The battery can be substantially recharged or “boosted” but in order to bring the battery to a fully charged condition, the charging cycle must be finished by charging at a low or normal rate. Some quick-chargers have a provision for finishing the charging cycle at a low rate so that the battery can be brought up to a fully charged condition.
Used with care, and employing all safeguards provided by the manufacturer, a quick-charger will not damage a battery which is in good condition. The “quick-charge” method must not be used, however, if the following conditions exist in the 1954 Buick battery.
- If gravity readings are not uniform, the low-reading cell may have an internal defect. Quick-charging may cause considerable heat to develop so that the battery would be ruined.
- If electrolyte is discolored with brownish sediment, quick-charging may produce an internal short and ruin the 1954 Buick battery.
- A sulphated battery will overheat during quick-charging. Such a battery requires charging at half the normal “slow-charge” rate for from 60 to 100 hours to reconvert the crystalline lead sulphate into active material again.
- A battery which has been badly overcharged may quickly fail if placed on quick charge.
- The battery cell voltages and the color of electrolyte should be checked a few minutes after the battery has been put on quick charge. If cell voltage readings are not uniform within 2/10 volt, or if electrolyte has become discolored with brownish sediment, quick-charging should be stopped immediately. Charging may be continued by the “slow-charge” method.

Leave A Comment
You must be logged in to post a comment.