Whenever we think of anything boiling, we instinctively think of it being hot. However, that is not true in every case. Just because water boils at 212° does not mean that all other substances will boil at the same temperature. Some would have to be put into a blast furnace to make them boil and give off vapor, such as aluminum, iron, etc. On the other hand, others will boil violently while setting on a cake of ice, such as ammonia, sulphurdioxide, freon, etc.
And so, each substance has its own peculiar boiling point temperature, but regardless of whether it is high or low, they all absorb unusually large quantities of heat without getting any warmer when they change from a liquid into a vapor.
Consequently any liquid, such as ammonia, that will boil at a temperature below the freezing point of water, will make ice cubes and keep vegetables cool in a mechanical refrigerator. In the early days of refrigeration, ammonia actually was one of the first and most popular refrigerants used. Today though, it is supplanted by better and safer man made refrigerants.
Nearly everyone is familiar with ammonia. Nearly every housewife uses it for housecleaning chores. lt makes our eyes smart and chokes us a little if we breathe any of it but other wise doesn’t seem very unusual. Actually, the so-called “ammonia” we know; is so highly diluted that it is practically all water. How ever if it were in its pure form, it would boil at 28° below zero!
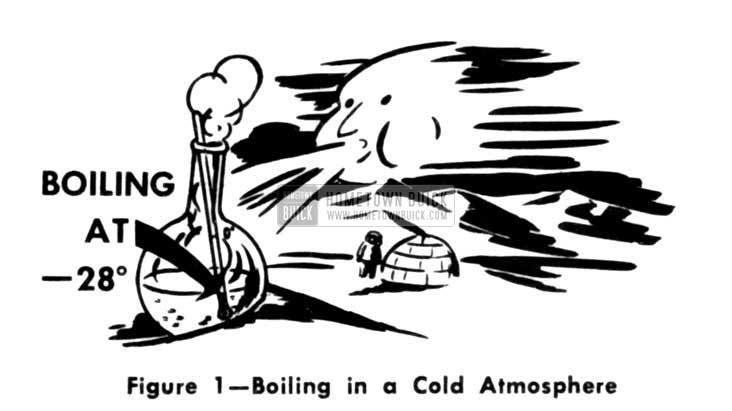
1953 Buick Boiling in a Cold Atmosphere
Maybe that does not mean very much until we picture a flask of ammonia setting on a cake of ice boiling away just like a tea kettle on a stove. No one would dare pick up a flask of pure ammonia with his bare hands because, even though boiling, it would be so cold and it would be drawing heat away from nearby objects so fast the human flesh would freeze in a very short time.
If we were to put a flask of ammonia inside a refrigerator cabinet it would boil and draw heat away from everything surrounding it as the transfer of temperature is always from a warm to a cooler object. So long as any ammonia remains in the flask it would keep on soaking up heat until the temperature got down to 28 degrees below zero. Now we can begin to see the similarity between a boiling tea kettle and a refrigerator. Ordinarily we think of the flame pushing heat into the tea kettle. Yet it is just as logical to turn our thinking around and picture the tea kettle pulling heat out of the flame. Both the tea kettle and the flask of ammonia do the same thing-they both draw in heat to boil, although they do so at different temperature levels.
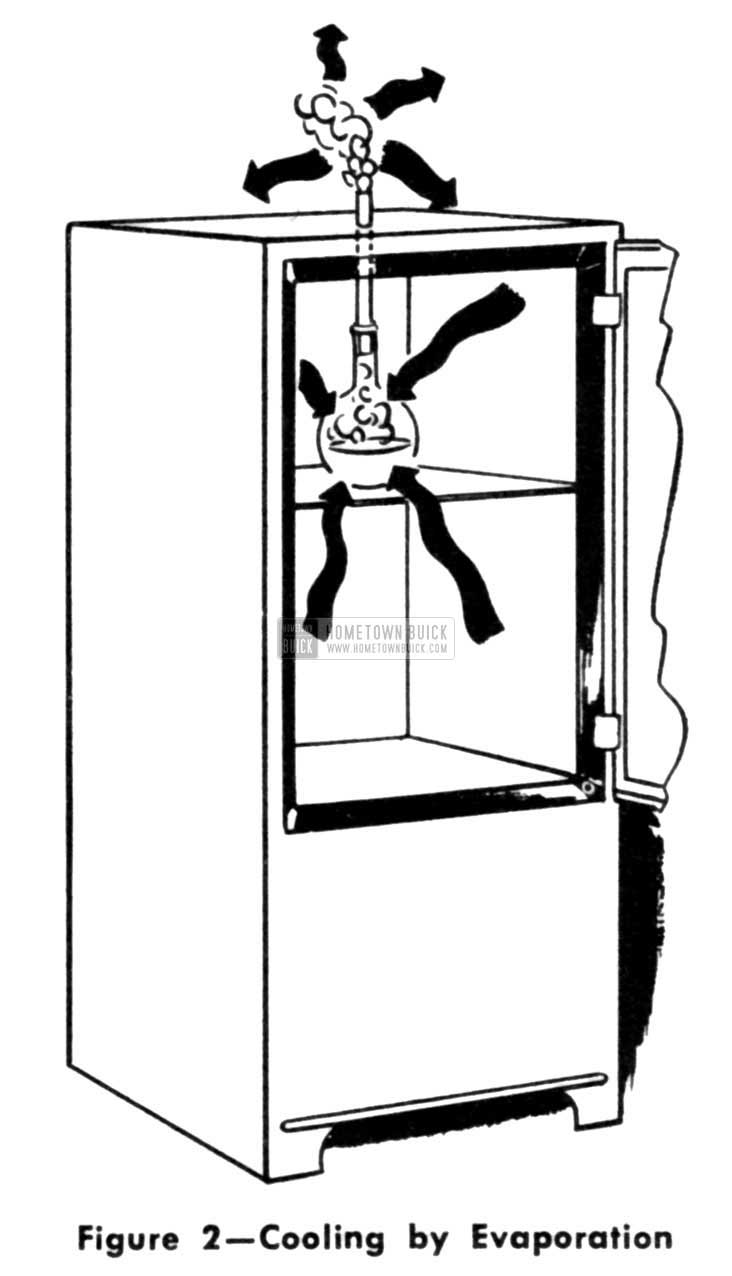
1953 Buick Cooling by Evaporation
There also is a similarity between the icebox and the mechanical refrigerator. In the former, water from melting ice literally carried heat out of the cabinet-now, rising vapors do the same job. (Fig. 3)
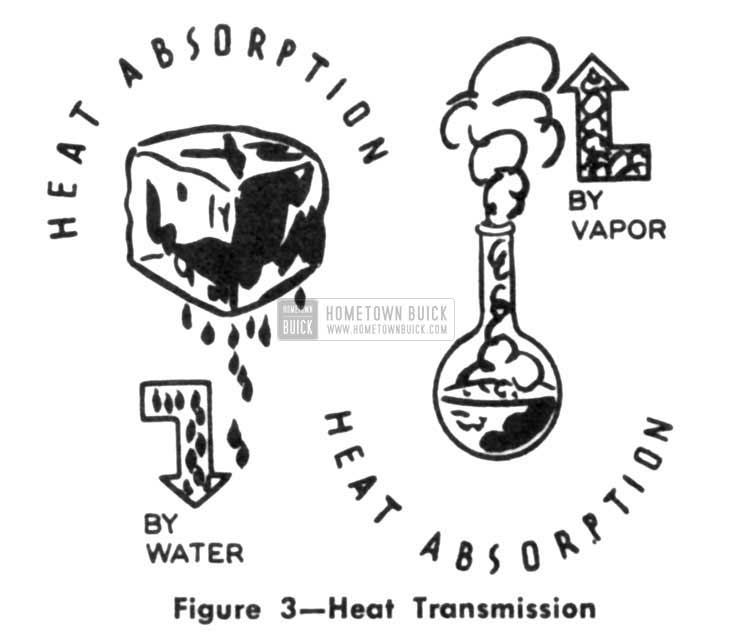
1953 Buick Heat Transmission
Of course, water was so cheap that we could afford to throw it away. But ammonia, or any other refrigerant, is too expensive to just let float away into the atmosphere. If there were some way to remove the heat from the vapor and change it into a liquid, it could be returned to the flask and used over again. (Fig. 4)
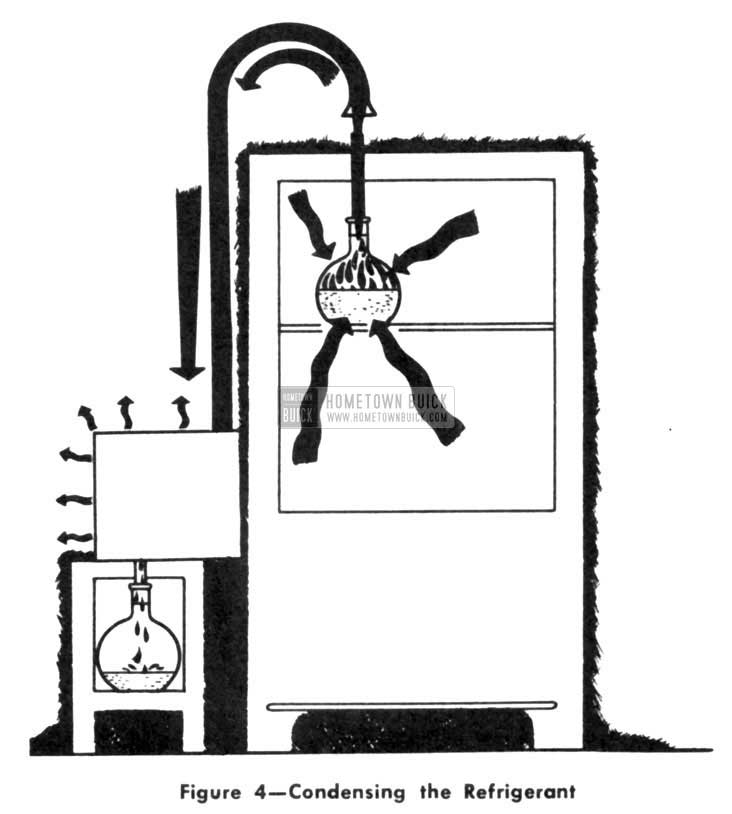
1953 Buick Condensing the Refrigerating System
There is a way, and that is where we find the biggest difference between the old icebox and the modern refrigerator. We used to put in new ice to replace that lost by melting. Now we use the same refrigerant over and over again.
Many years ago when two scientists started experiments with pressure they were interested merely in condensing vapor. But soon they began to notice some strange things happening. They already knew that each substance would condense at the same temperature at which it boiled. This temperature point was a clear cut division like a fence. On one side, a substance would be liquid. lmmediately on the other side, it would be a vapor. Whichever way the substance would go, from hot to cold, or cold to hot, it would change its character the moment it crossed the fence. (Fig. 5)
1953 Buick Condensing and Boiling Point
But pressure seemed to move the fence! Water, they knew for example, would boil at 212 degrees. Naturally, they expected the steam to condense at the same temperature. But when ever they put pressure on steam, it didn’t! It would condense at some temperature higher than 212 degrees. The greater the pressure, the higher the temperature at which a vapor would condense. This effect of pressure on the condensing point of vapors was just a laboratory curiosity to the scientists, but a modern electric refrigerator would not work without pressure and here is why: We know that ammonia, for example boils or vaporizes at 28 degrees below zero. A thermometer will show us that the rising vapors, even though they have soaked up lots of heat, are only lightly warmer. (Fig. 6)

1953 Buick Vapor-Liquid Relationship
But the vapors must be made warmer than the room air if we expect heat to flow out of them. Also, the condensing point temperature must be above that of room air or else the vapors won’t condense. This is where pressure comes to the rescue. With pressure, we can compress the vapor, thereby concentrating the heat it contains. When we concentrate heat in a vapor that way, we increase the intensity of the heat or, in other words, we increase the temperature. The most amazing part of it all is that we made the vapor hotter without actually adding any additional quantity of heat. (Fig. 7)
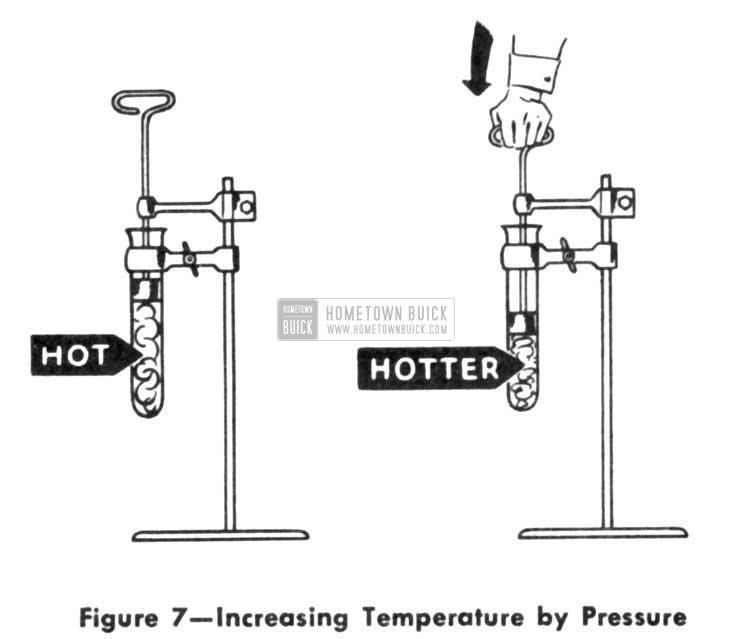
1953 Buick Increasing Temperature by Pressure
We have now covered all the scientific ground rules that apply to refrigeration. Most likely they still are a little hazy, but it is easy enough to remember these main points: all liquids soak up lots of heat without getting any warmer when they boil into a vapor, and, we can use pressure to make the vapor condense back into a liquid so it can be used over again. With just that amount of scientific knowledge here is how we can build a refrigerator. (Fig. 8)
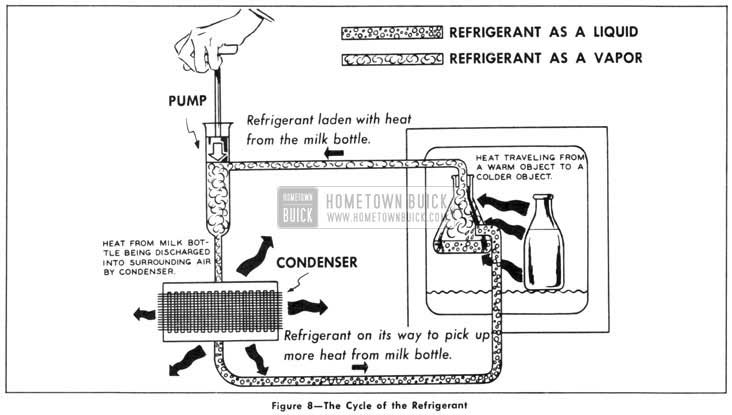
1953 Buick The Cycle of the Refrigerant
We can place a flask of refrigerant in an icebox and know it will boil at a very cold temperature and will draw heat away from everything inside the cabinet.
We can pipe the rising vapors outside the cabinet and thus provide a way for carrying the heat out. Once we get the vapor outside, we can compress it with a pump. With enough pressure, we can squeeze the heat out of “cold” vapor even in a warm room.
By removing the heat and making the refrigerant into a liquid, it becomes the same as it was before. So, we can run another pipe back into the cabinet and return the refrigerant to the flask to be used over again.
When we Iook inside a typical home mechanical refrigerator, we will always find a small separate compartment in the upper portion of the food storage space. It is the one that contains the ice cubes and which usually is covered on the outside with a coating of frost.
This is known as the “freezer,” “evaporator,” or as we shall refer to it in the automobiles the “cooling coil.” It does the same job as the flask of ammonia we previously spoke about. (Fig. 9)
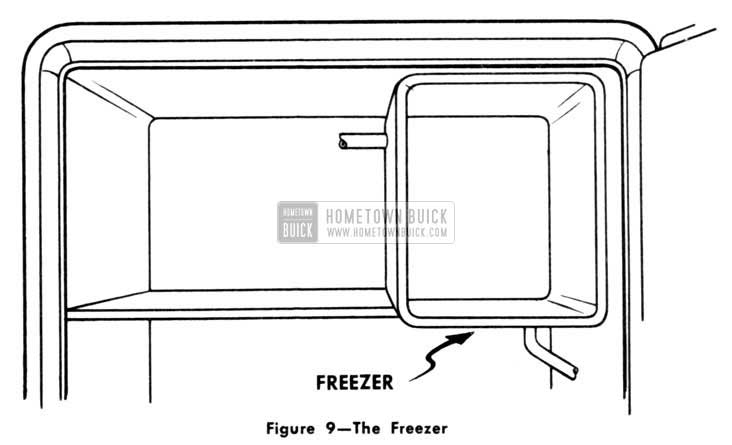
1953 Buick Freezer
Actually, the freezer is a double walled tank. Between the walls of this tank there are tubular or channel-like spaces containing the refrigerant. It may be sulphurdioxide but more likely is one of the modern freon refrigerants.
If you Iisten closely, after the motor has stopped running and when it is quiet in the house, you usually can hear a faint gurgling noise inside the freezer. That is the sound of the refrigerant boiling. In boiling, the refrigerant absorbs heat and changes into a vapor. By piping this vapor outside the cabinet, we can carry out the heat that caused its creation.
Generally, you can find two or three pipes leading away from the freezer. Sometimes they are so well concealed that they can’t easily be seen, but nevertheless they are there. The largest of these pipes is the one that carries the refrigerant vapor outside the cabinet. This, then, is the path through which heat is carried out.
Once we get the vapor out of the box, all we have to do is remove the heat it contains. Since heat is the only thing that expanded the refrigerant from a liquid to a vapor in the first place, removal of that same heat will Iet the vapor condense into a liquid again. Then we can return the liquid refrigerant to the freezer to be used over again.
Actually, the vapor coming out of the cabinet is very cold. We know the liquid refrigerant boils at temperatures considerably below freezing and that the vapors arising from it are only a shade warmer even though they do contain quantities of heat. Consequently, we can’t expect to remove heat from sub-freezing vapors by “cooling” them into room air temperatures that usually range between 60 degrees and 100 degrees-heat refuses to flow from a cold object toward a warmer object.
But, with a pump we can squeeze the heated vapor into a smaller space. And, when we com- press the vapor, we also concentrate the heat it hotter without adding any heat. Then we can cool it in comparatively warm room air. This is the only responsibility of a compressor in a refrigerating system. (Fig. 10)
1953 Buick Compressor
It is not intended to be a pump for circulating the refrigerant. Rather, its job is to exert pressure for two reasons : (1) Pressure makes the vapor hot enough to cool off in warm room air. (2) At the same time, the compressor raises point at the temperature of the room air so it will condense. is still a vapor although it is now quite hot and ready to give up the heat it has carried out of the cabinet. One of the easiest ways to help refrigerant vapor discharge its heat is to send it through a radiator-like contrivance known as a condenser. (Fig. 11)
1953 Buick Condenser
The condenser really is a very simple device having no moving parts. It does exactly the same job as the familiar radiator in a typical home steam-heating system. There, the steam is nothing more than water vapor. In passing through the radiator, the steam gives up its heat and condenses back into water. The same action takes place in a refrigerator condenser. The refrigerant vapor gives up its heat, which is quickly and easily radiated into the surrounding room air through the large :finned surfaces of the condenser. In giving up its heat, the refrigerant vapor condenses back into a liquid which collects in a pool at the bottom of the condenser. (Fig. 12)
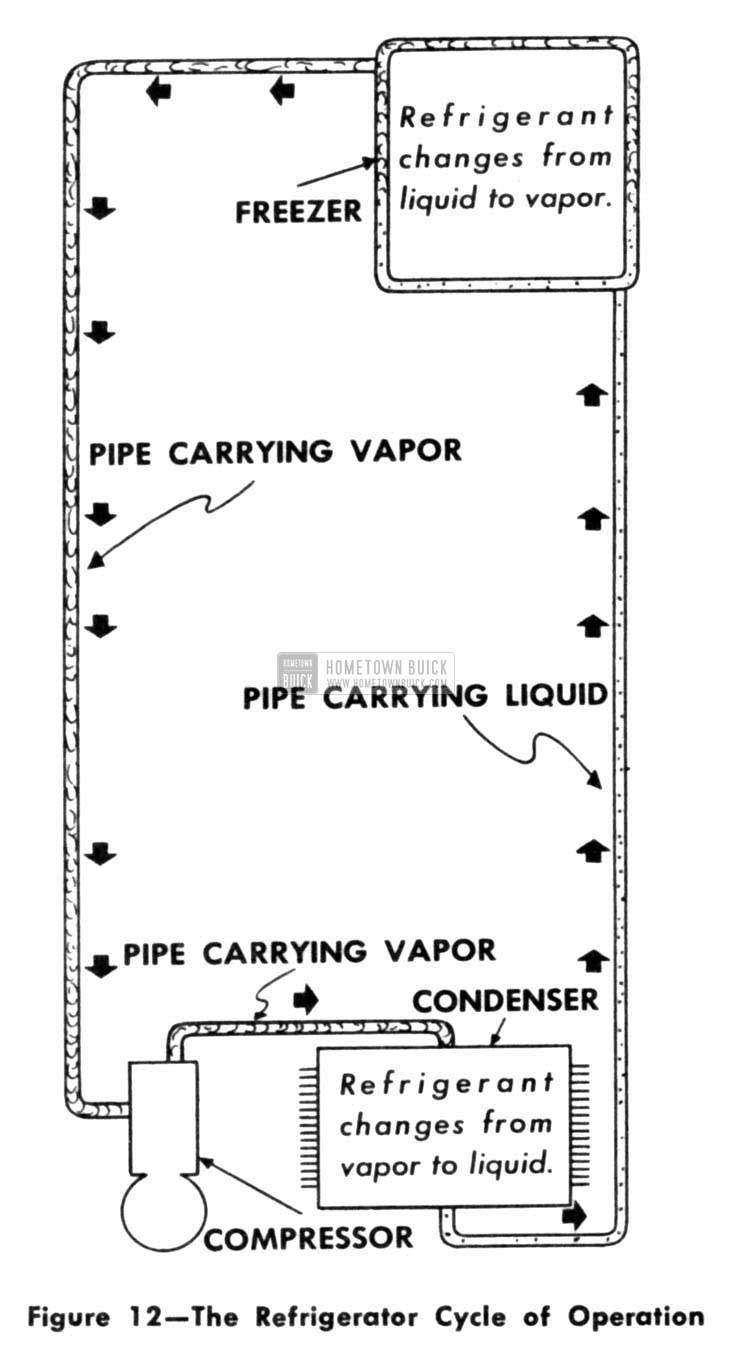
1953 Buick Refrigerator Cycle of Operation
As we said before, when the refrigerant condenses into a liquid, it again is ready for boiling in the freezer. So we can run a pipe from the condenser back to the freezer. This, then, accounts for the medium-size pipe connected to the freezer. The smallest of the three pipes, if there happens to be a third one, is part of the temperature control unit. However, for the time being, we’ll ignore it and save it for later discussion. These three units then-the freezer, the compressor, and the condenser-are the main working parts in any typical refrigeration system (including the automobile conditioner). We have the freezer inside the cabinet where the refrigerant boils and changes into a vapor absorbing heat as it does so. We have the pump or compressor to put pressure on the refrigerant so it can get rid of its heat. And we have a condenser outside the box to help discharge the heat into the surrounding room air. (Fig. 13)
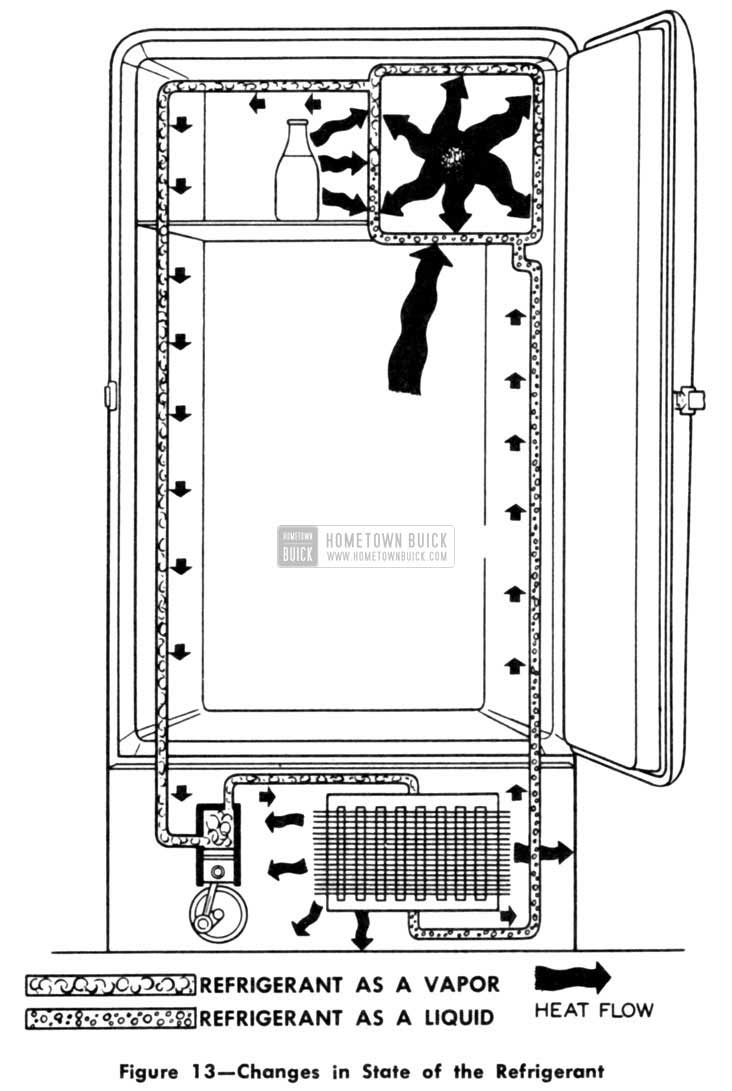
1953 Buick Changes in State of the Refrigerant
There is one more unit that cooperates with these three. It doesn’t do any real work but it does act as a sort of traffic officer in controlling the flow of the refrigerant through the system. To get a better idea of what this unit does, let’s first do a little experimenting with an ordinary tire pump.
When we use a tire pump to inflate an auto mobile tire, we are exerting pressure only be cause we are “pushing” against the air already entrapped inside the tire. If you question this, just try pumping up a tire that has a puncture in it. You could pump all day, and still not be able to build up any pressure. (Fig. 14)
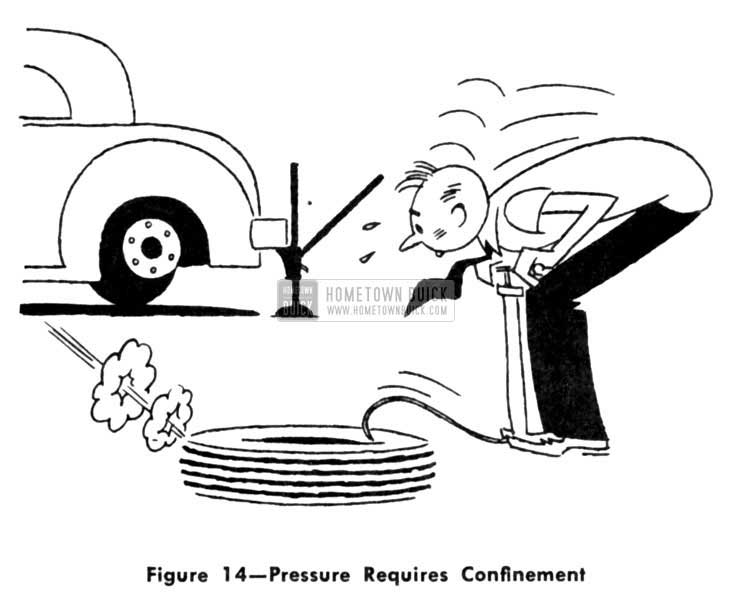
1953 Buick Pressure Requires Confinement
As fast as you would pump the air in, it would leak out through the puncture. About all you would be doing would be circulating fresh air through the tire. Unless you have something to push against-to block the flow of air-you can’t create more than a mere semblance of pressure.
The same situation holds true in a refrigerator system. The compressor can pump refrigerant vapor through the system, but unless it has something to push against, it cannot build up pressure. All the compressor would be doing would be to circulate the vapor without increasing its pressure.
Yet, in a refrigeration system, we can’t just block the flow through the system entirely. All we want to do is put pressure on the refrigerant vapor so it will condense at normal room temperatures. What’s more, this must be done some time after the vapor leaves the freezer and be fore it returns as a liquid. We can’t have excessive pressure in the freezer because that would slow down the boiling of the refrigerant and thus penalize the refrigerating effect.
There are several ways pressure and flow can be controlled. It can be done with float valves, or with a coil of fine tubing known as a capillary. The first are used mostly on large commercial and apartment house refrigerating systems. The latter usually is used only on home refrigerators, food-freezers, and small commercial units. Also there are some rather complicated pressure-regulating valves, and it is this type of control that is used in our auto mobile air conditioning.
For a better understanding of a control valve Iet us describe its action and function. (Fig.15)
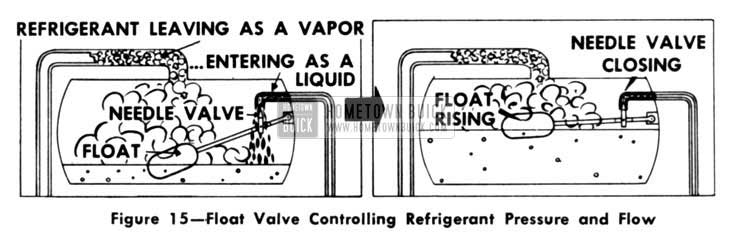
1953 Buick Float Valve Controlling Refrigerant Pressure and Flow
Sometimes, a float valve is located inside the freezer. It consists simply of a float that rides on the surface of the liquid refrigerant. As the refrigerant liquid boils and passes off as a vapor, the liquid Ievel drops lower.
By means of a simple system of mechanical linkage, the downward movement of the float opens a valve to Iet refrigerant in. The incoming liquid raises the fluid Ievel and, of course, the float rides up along with it.
The liquid Ievel falls rather slowly as the refrigerant vaporizes. Likewise, the float goes down gradually and gradually opens the valve just a crack. New refrigerant liquid barely seeps in through the “cracked” valve.
Under normal operating conditions, that is actually what happens. So long as the compressor is running, there is a continuous flow of refrigerant through the system. Yet, at the same time, the flow is restricted sufficiently to enable the compressor to exert pressure on the vapor.
The “Capillary Tube” is by far the simplest device for controlling pressure. It is nothing more than a long piece of fine tubing which in some cases is coiled to save space. There are no moving parts to wear or get out of order – so, of course, there are no valves to close for building up pressure or to open to Iet refrigerant flow. (Fig. 16)
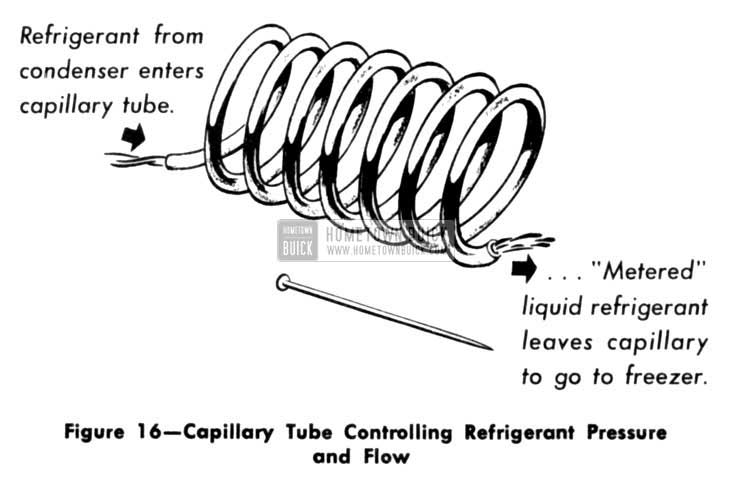
1953 Buick Capillary Tube Controlling Refrigerant Pressure and Flow
However, by proper design of size and length, the capillary restrictor resists the flow of refrigerant in vapor form so that the compressor can build up enough pressure to make the vapor condense. At the same time, the capillary restrictor Iets liquid refrigerant through with less resistance. It can do these two jobs simultaneously because liquids are much more concentrated and compact than vapors. They behave like a well-trained line of marching soldiers passing through a tunnel. Vapors, on the other hand, wander and surge like an unruly crowd … consequently, they can’t get through the tunnel as quickly or easily. Thus, the capillary restrictor holds back refrigerant vapors so they can be compressed and condensed, but Iets refrigerant liquids through more easily.
Whether the refrigerating system uses the “Float Valve,” “Capillary” or “Pressure Regulating Valve,” it is at this unit the refrigerant is relieved of its pressure and then is in a state to once again vaporize in the cooling coil.
So far, we’ve discussed only what each unit in a refrigerating system does. We’ve learned that the freezer is the unit in which liquid refrigerant soaks up heat from inside the cabinet, the compressor is a pump for squeezing this heat out of the vapor, the condenser is a radiator for getting rid of the heat, and the control valve is a device for regulating the pressure on the refrigerant.
Now, let us step into the next phase of the program and have a good look at the various components as used by Buick.
Although presently we will not be required to disassemble and assemble the various parts, you no doubt will be interested in knowing their construction and, very definitely, how each operates.

Leave A Comment
You must be logged in to post a comment.